Projects
Reference | CA20121
Execution date | 19/10/2021 – 18/10/2025
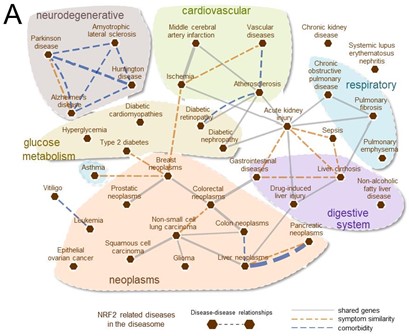
Non-communicable diseases (NCDs) account for 77% of all deaths in Europe and remain the most prevalent and without effective therapy.
The transcription factor NRF2 is a master regulator of multiple cytoprotective responses and a key molecular link among many NCDs. It provides a unique strategy for drug development and repurposing that is now starting to be translated to the pharmacological and clinical arena.
This Action will build a network of excellence for integrating and spreading the existing knowledge and providing innovative services, drugs and tools related to NRF2-pharmacology, with the final goal of boosting the translation to the European industry sector.

Development of NRF2-activating drugs for innovative therapies for Alzheimer's disease
Reference | S2017/BMD-3827
Execution date | 01/01/2018 – 30/06/2022
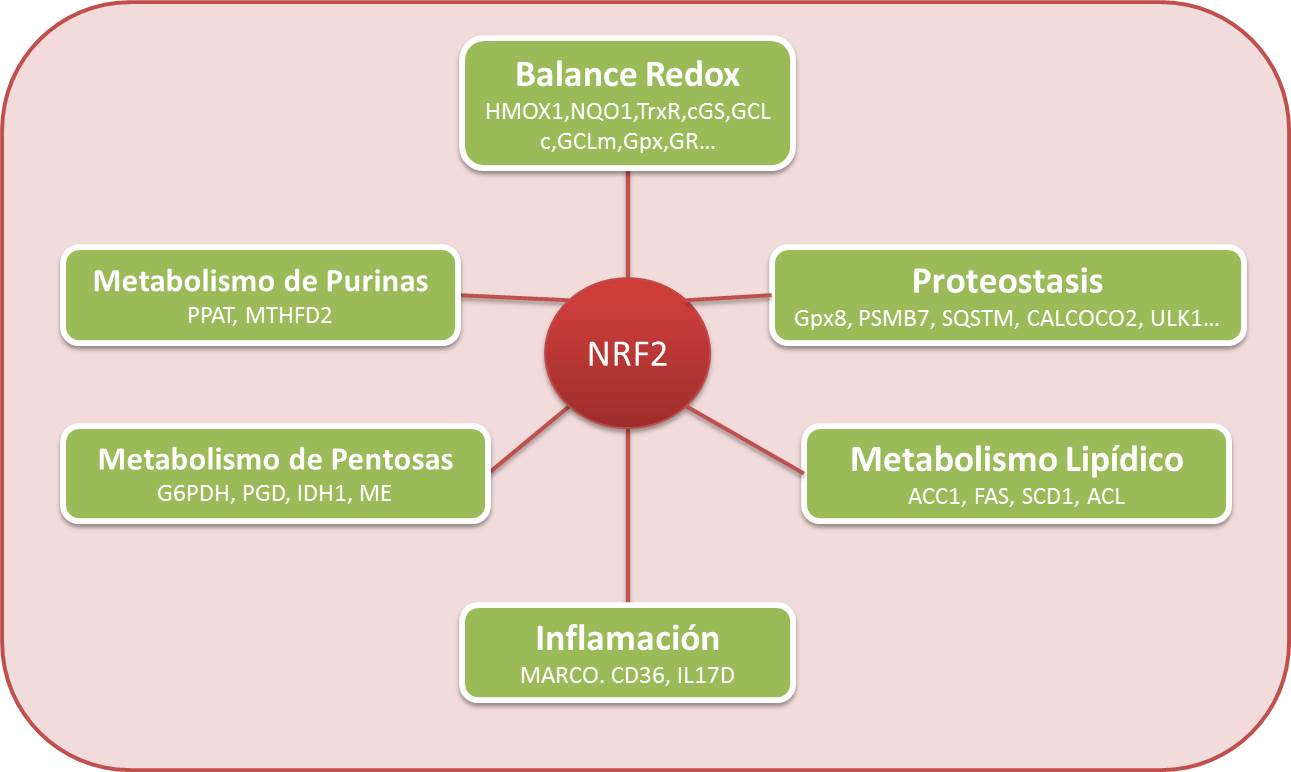



Interplay between neuroinflammation, oxidative stress and proteostasis: new targets to tackle neurodegeneration in AD (NewTargets4AD)
Reference | RTI2018-095793-B-I00
Execution date | 01/01/2018 – 30/06/2022


Imagen
RED2018‐102362‐T. Red de investigación traslacional sobre la regulación farmacológica de NRF2 en enfermedades no transmisibles. MINECO. PI. Antonio Cuadrado. UAM. 01/12/2019-1/12/2020.
SAF2015-63935R. Exploración del eje alfa-7nAChR/Nrf2/HO-1 en la interacción microglía-neurona y su impacto en los procesos de neurodegeneración y neuroprotección. MINECO. PI: Manuela G. López. UAM. 01/01/2016 – 31/12/2018.
COST Action BM1203:EU-ROS. EU-ROS: The European Network on Oxidative Stress and Redox Biology Research. Coordinador: Andreas Daiber. 05/12/2012-04/12/2016. Manuela G. López. Miembro sustituto del Comité ejecutivo en representación de España.
SAF2012-2332.Control colinérgico de la neuroinflamación y su participación en la neuroprotección. MINECO. PI: Manuela G. López. 01/01/2013-31/12/2015.
SAF2009-12150. Contribución de los receptores nicotínicos a la neuroprotección y a la neuroinflamación. MICINN. PI: Manuela G. López. 01/01/2010 – 31/12/2012.





